Research ProjectParasites in our Backyard
Parasites in our Backyard
Affiliated Labs
Project Goal
To identify parasites that reside in the Rhode River, track yearly changes in diversity and abundance, and determine their impacts on ecosystem function, population dynamics, and trophic interactions.
Description
Researchers at SERC have been examining population and community fluctuations in the Rhode River for multiple decades. Since the Coastal Disease Ecology Lab (formerly the Marine Disease Ecology Lab) began in 2017, we have been incorporating parasites into these surveys, which include hosts from benthic and nearshore habitats and oyster beds. We are identifying parasites from potential hosts (i.e., grass shrimp, mummichogs, snails, oysters, clams) collected from these surveys and recording the prevalence, diversity, and abundance of each parasite found. Additionally, we conduct lab and field experiments to understand the various impacts these parasites have on the host populations and communities with in Rhode River ecosystem.
Interns and volunteers interested in working with the Coastal Disease Ecology Lab will participate in these surveys. The fieldwork involves collecting hosts through seining, trawling, sediment cores, and water samples. Laboratory techniques typically include microscopic dissections and genetic methods. If you are interested in a summer internship to work on this project, please apply here. If you are interested in volunteering to work on this project, please apply here.

Grass shrimp are small, transparent crustaceans with a distinct serrated rostrum. They can be found in the western Atlantic Ocean to the Gulf of Mexico, including shallow waters throughout the Chesapeake Bay. Within the Chesapeake Bay there are three native (Palaemonetes pugio, P. intermedius, P. vulgaris) and one invasive species (Palaemon macrodactylus) of grass shrimp. These shrimp live approximately 1 year and breed in the summer months with females more easily spotted as they carry eggs in a large brood pouch. Grass shrimp are foragers that eat detritus, worms, algae, and other small crustaceans. They are also an important prey species for several small foraging fish (ex. mummichogs), blue crabs, and striped bass. Within the Chesapeake Bay, grass shrimp a dominant force in the transfer of energy and parasites through the food web.
Our lab collaborates with the Marine Invasions Research Lab to examine the impact of two common parasites, the trematode Microphallus turgidus and the bopyrid isopod Probopyrus pandalicola, on shrimp population dynamics. The metacercaria life stage of some trematodes infect these shrimp and can be easily spotted in the clear tissues of the host. We have seen shrimp infections ranging from 1 to 84 metacercaria! Cercaria infect grass shrimp after leaving a snail host, then develop into metacercaria within shrimp muscle tissues. Parasitized fish undergo behavior changes increasing the likelihood of predation. When shrimp are consumed by fish, the parasite moves up a trophic level and metacercaria ultimately develop into adult trematodes, the final reproductive stage of the parasite. While the shrimp is a host for trematodes in the middle of their life cycle, it is the final host in the life cycle of the bopyrid isopod, which are macroscopically visible ectoparasitic isopods that live in the gill cavity under the shrimp’s carapace. Grass shrimp become infected by consuming copepods that are infected. The first larvae to parasitize a host develops into a female while the second becomes male. Bopyrids have more direct negative influences on shrimp host populations as they affect shrimp respiration, camouflage from predators, behavior, and reproductive output, just to name a few!
Mummichogs (Fundulus heteroclitus) are small sexually dimorphic forage fish found along the Western Atlantic and Gulf of Mexico. During summer they occupy shallow areas in marshes, grass flats, and creeks from a few yards to 100 feet offshore, and can be found in deeper waters or mud burrows during winter months. During breeding season from April to August males sport bright yellow fin tips. Mummichogs can live up to three years and reach sexual maturity during their second year. They are opportunistic omnivores preyed upon by larger fish & wading/seabirds. Their diet is vast and can include algae, plants, insects & larvae, worms, crustaceans, snails, fish & their eggs. Not only are mummichogs a key prey of commercially valuable fish, such as striped bass, but they are hosts to a diverse group of parasites!
Our lab collaborates with the Fisheries Conservation Laboratory to acquire and examine the skin, body cavity, liver, intestine, muscle, and gills of mummichogs with the goal of assessing total abundance and biodiversity of parasites over time. Parasites found include nematodes, cestodes, monogenean and digenean trematodes, leeches (Myzobdella lugubris), copepods (Ergasilidae manticus, Argulus sp.) and a plethora of cysts & larval life stages.

Hydrobiid snails are tiny aquatic operculate gastropods found in fresh and brackish waters. These snails are quite cryptic, measuring less than 8mm and often found buried in the mud! Within the Chesapeake Bay, Litterodinops monroensis, are found along muddy bottoms on the fringe of marsh habitat where there is plenty of detritus, algae, and diatoms to eat.
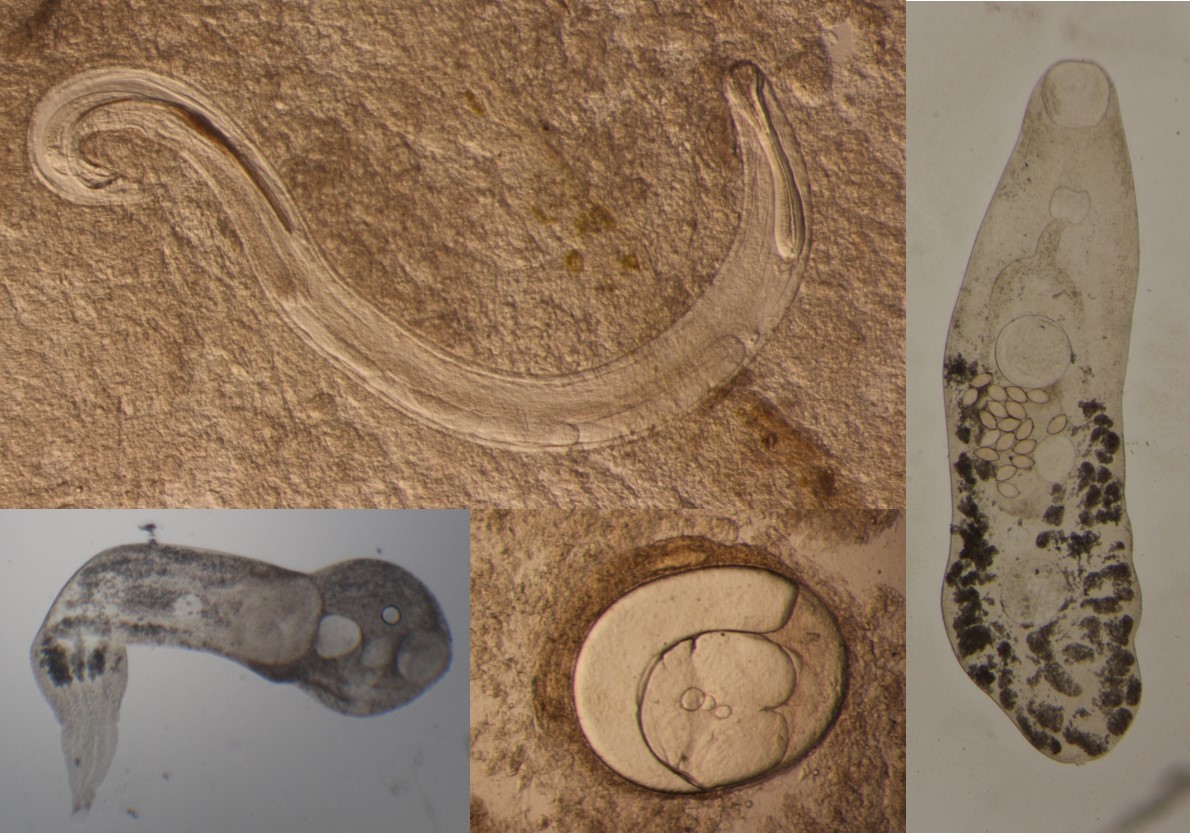
Our lab collects and screens snails for infections from a parasitic worm. The stage of the worm that infects snails is called the cercaria, an early stage in the life cycle of trematode worms that doesn’t look much like a traditional worm. Trematodes have really complicated life cycles that include multiple hosts. Even within the vast group of trematodes, there are differences in life cycle complexity, number and development of life stages, and number and/or type of hosts. For example, a general trematode life cycle progression could be eggs laid by adult trematodes hatch and develop into miracidium which infect the first intermediate host, in this case a hydrobiid snail. Within the snail, miracidium develop into sporocysts and produce more sporocysts that undergo transformation and produce cercaria. Once cercaria numbers within an infected snail host become significant, cercaria explode out of the snail’s shell and seek out another host, the second intermediate host, to continue their life cycle. This second host could be a shrimp or fish and is the place cercaria develop into metacercaria. The lifecycle of trematodes ends in the definitive host where metacercaria develop into adult trematodes. In some trematode species, the second host is the definitive host with cercaria developing directly into adult trematodes. Studying cercaria infections in snails helps us understand trophic transfer of trematodes within an ecosystem.


Clam (Macoma balthica)
Macoma balthica (Baltic clam) is a small burrowing bivalve with a thin milky white shell. They are one of several bivalve species found within the Chesapeake Bay and can grow up to 1.5 inches. Macoma spp. are plankton feeders equipped with a long siphon for sucking in debris and plankton, and a short siphon for ejecting waste. They are a sought-after snack for blue crabs and eels. Benthic clams were found to make up the highest percentage of adult blue crab diet and about 30% of juvenile crab diets. Adult crabs can eat up to 7 clams a day! Clams can escape predation pressure and detection by burrowing deeper, as deep as 30cm, in muddy bottoms!

Oyster (Crassostrea virginica)
The eastern oyster is a hard-shelled bivalve found in brackish and saltwater ecosystems along the eastern seaboard. Oysters can live up to 20 years in captivity and continue to grow throughout their lifetime! They are filter feeders that consume phytoplankton (algae) as water passes along their four rows of gills. Oysters typically grow an inch per year based on salinity and overall water quality, reaching market size in about 3 years. As larvae they are food for other filter feeders, especially ctenophores. As juveniles and adults, they are prey for Bay species like cownose rays, black drum, and oyster drills (predatory snail). Crabs, worms, boring sponges, disease, and stress due to poor water quality are also sources of mortality for adult oysters. In addition to maintaining bay cleanliness and clarity through filtering water, oysters serve as important ecosystem engineers by creating habitat for a vast number of critters! Humans recreationally benefit from services provided by oysters but also economically benefit from the oyster fisheries and other commercial fisheries, like striped bass, that are supported by the ecosystem services provided by oyster reefs.
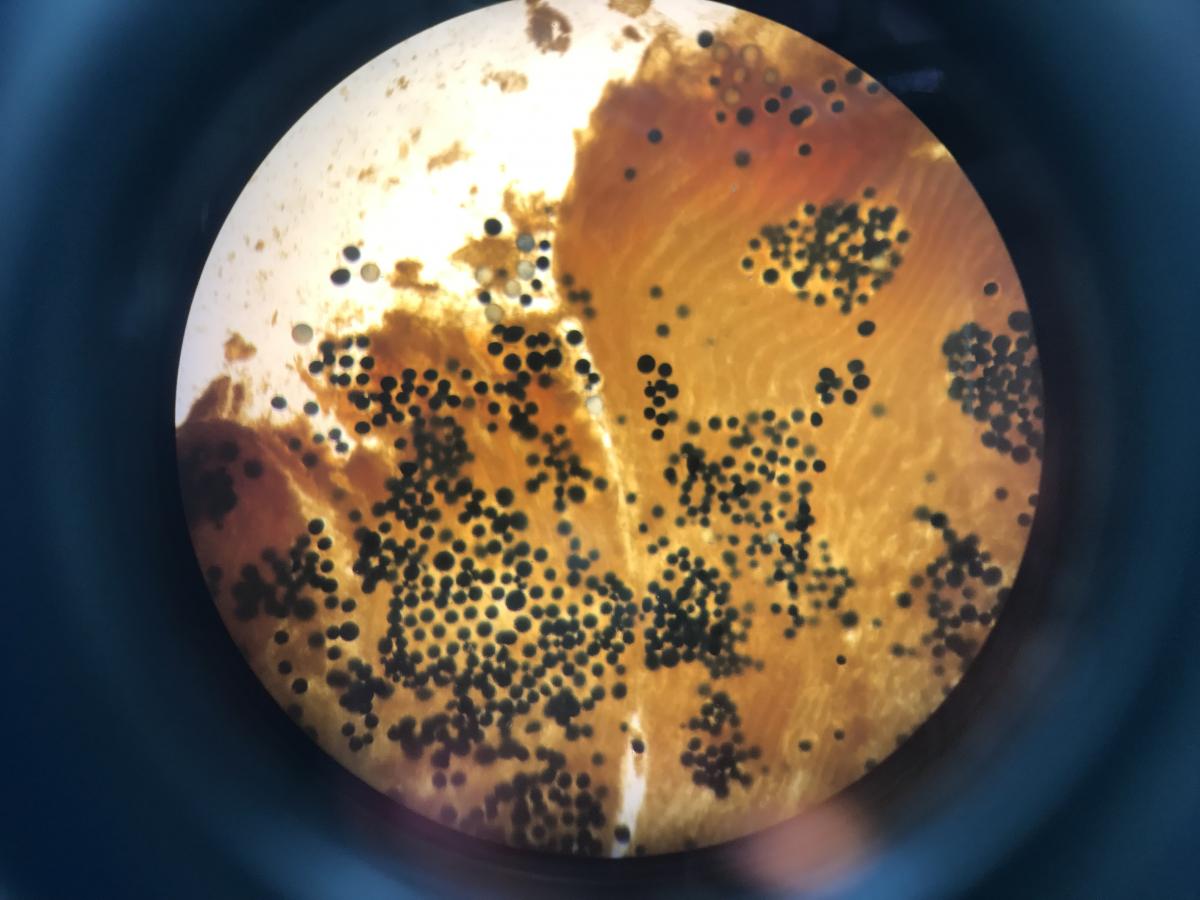
Our lab samples bivalve populations within the Rhode, West, and South Rivers to assess the prevalence and intensity of the parasites Mytilicola spp. and Perkinsus spp. Mytilicola spp. are copepods that live inside the intestinal tract of unsuspecting bivalves. Multiple species are found across North America, Europe and Asia, and infect multiple bivalve hosts including clams, mussels, and oysters. Visible to the naked eye, you can identify Mytilicola by their charismatic, fire-engine red color. Females are larger in size than males and develop egg sacks. Females can reach 12mm in size and males 5mm. Usually less than 10 individuals live inside a single host. Mytilicola are considered serious pests by some, but the extent of damage and pathological effects to their hosts are debated. Perkinsus species are protistan parasites found worldwide that infect oysters, mussels, and clams. Perkinsus was first discovered in Chesapeake Bay oysters (Crassostrea virginica) in 1949. Within Chesapeake Bay two species exhibit host & salinity preference with Perkinsus marinus infecting and causing “Dermo” disease in oysters at a higher salinity, and P. chesapeaki infecting clams at a lower salinity. All stages of Perkinsus development are infective. The general life cycle of Perkinsus spp. begins when zoospores are filtered out of the water and internally develop into trophozoites, displacing host tissue cells and stealing nutrients. Trophozoites continue to grow, multiply, and shed from host tissues eventually making it into the water column. We perform Ray’s Fluid Thioglycollate Medium (RFTM) assays of clam palp tissue and oyster mantle & anal tissue and clam palps, staining tissues with iodine to count trophozoite density as an indicator of disease intensity. RFTM media provides trophozoites with nutrients so they enlarge and develop a thick cell wall that is easily stained with iodine. Infection intensity within a host is determined by parasitic cell counts in the tissues. Infections are more intense May through October when parasites are growing and multiplying and can lay dormant over winter until warmer temperatures return. Bivalves die from Perkinsus infections as a result of malnutrition and stress, a process that can take up to 3 years!